Facts About Superoxide Dismutases: Role In Redox Signaling Revealed
The SODs represent the first enzymatic defense system against radical damage by oxygen: thus, this enzyme is essential for all aerobic organisms, but not for anaerobes. In support of this hypothesis, McCord believed that the existence of an aerobic organism depends mainly on its ability to produce SODs since its deficiency is responsible for oxygen sensitivity and allows survival only in an anaerobic environment.
In physiological conditions, the superoxide dismutases, together with the non-enzymatic ROS scavengers as vitamins E, A, and C maintain a steady state between oxidant and antioxidant systems (Russo et al., 2011). The dysregulation in redox homeostasis, determined by an imbalance between ROS production and scavenging capacity, determines considerable cellular damage as membrane lipoperoxidation, nucleic acid and structural alterations of proteins contributing to neurodegenerative and cardiovascular diseases.
In the last years, many data obtained in in vitro studies performed in many cellular lines, mainly neuroblastoma SK-N-BE cells, indicate that SOD1 is secreted and is able to activate, through muscarinic M1 receptor, cellular pathways involving ERK1/2 and AKT activation; these effects are associated with intracellular calcium increase that is further accentuated when these cells are stimulated with mutated SOD1G93A.
The intracellular cytosolic SOD1 localization has been a matter of debate; recent evidences, performed in transfected mouse neuroblastoma neuro2 cells, demonstrated that both wild type SOD1 (wt-SOD1) and SOD1 mutants are distributed into luminal structures of endoplasmic and Golgi apparatus (Urushitani et al., 2008). The first experimental evidence that some cellular lines could be able to secrete the Cu,Zn superoxide dismutase date back to many years ago when we, for the first time, showed the secretion of this protein by experiments performed in hepatocytes and fibroblasts (Mondola et al., 1996), neuroblastoma SK-N-BE cells (Mondola et al., 1998; Gomes et al., 2007; Polazzi et al., 2013) and in thymus derived epithelial cells (Cimini et al., 2002).
In addition, we demonstrated that in human neuroblastoma SK-N-BE cells, that show a greater sensitivity to glucose deprivation-induced cytotoxicity due to enhanced sensitivity to ROS (Shutt et al., 2010), SOD1 export takes place in normal conditions and is increased following oxidative stress (Mondola et al., 1996, 1998). Successively, we showed that SOD1 in human neuroblastoma SK-N-BE cells is exported through a microvesicular secretory pathway that is impaired by brefeldin-A (BFA), and by 2-deoxyglucose, and sodium azide, which reduces ATP intracellular pool (Mondola et al., 2003).
Indicators on The Role Of Manganese Superoxide Dismutase In … – Hindawi You Need To Know
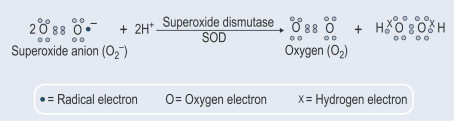

Another important aspect was the discovery that besides the constitutive SOD1 export, the secretion of this enzyme is also induced. To this respect, we showed (Santillo et al., 2007) that SOD1 is actively released from rat brain synaptosomes as well as from rat pituitary GH3 cells that represent a good model to study the inducible SOD1 release since they possess all the neuronal protein machinery involved in synaptic vesicle exocytosis.
In addition, in the attempt to evaluate the possible role carried out by SOD1 export, we recently demonstrated, in SK-N-BE neuroblastoma cell line, that this enzyme is able, through the involvement of muscarinic M1 receptor, to activate ERK1/2 and AKT in a dose and time-dependent manner. This effect was remarkably reduced by M1 receptor silencing as well as by using M1 antagonist pirenzepine (Damiano et al., 2013).
However, FGF-1 and the 18 kDa isoform of FGF-2 have been shown to be secreted by an alternative pathway being directly translocated from the cytoplasm into the extracellular space. Analogously, also interleukin 1β (IL-1β) has been reported to be secreted by a vesicular non-classical export pathway. Soluble proteins classically contain N-terminal signal peptides that direct them to the translocation apparatus of the Endoplasmic Reticulum (ER) (Walter et al., 1984).
In addition, non-classical protein secretion is both energy and temperature dependent and can be stimulated or inhibited by various treatments (Cleves, 1997; Hughes, 1999). The list of proteins that could be exported from cells in the absence of a functional ERG system (unconventional secretory pathway), as IL-1β and galectin-1 (also referred to as L-14), is continually growing; for further data see the review of Nickel (2003).

For this reason SOD1 secretion should bypass the canonical ERG secretory pathway. We previously demonstrated that BFA as well as 2-deoxyglucose and sodium azide (NaN3), impairs SOD1 export (Mondola et al., 2003). In our opinion, the treatment with BFA probably dysregulates not only the classical secretory ERG pathway but also the microvesicular membrane traffic of unconventional protein secretion or alternative protein export.
The 20-Second Trick For Superoxide Dismutase: Health Benefits, Uses, Side Effects …
Furthermore, these authors showed that SOD1A4V inhibits secretory protein transport from the ER to the Golgi apparatus. Amyotrophic lateral sclerosis (ALS) is an adult onset, neurodegenerative disease characterized by selective death of the upper and lower motor neurons of the brain and spinal cord. Symptoms include muscle atrophy, spasticity, paralysis and eventual death from respiratory failure within 3–5 years of diagnosis.
Clinical and pathological processes indicate that ER stress represents a key pathway involved in cell death. In the transgenic SOD1G93A ALS rat model unfolded protein response and ER stress-induced apoptosis has been observed (Atkin et al., 2006); an unfolded protein response, including induction of stress sensor kinases, chaperones, and apoptotic mediators, has been shown also in spinal cord motor neurons of human patients with the sporadic form of ALS (sALS) that is not restricted to SOD1 mutations (Atkin et al., 2008).
SOD1 and other proteins are misfolded in fALS and in sALS, but it is not clear how this triggers ER stress, fragmentation of the Golgi apparatus, disruption of axonal transport and apoptosis. Nearly 20% of fALS is caused by SOD1 gene mutations (Neumann et al., 2006). Indeed, the majority of SOD1 mutants maintain their enzymatic activity suggesting the occurrence of gain of toxic activity function rather than a simple loss of function (Strong et al., 2005; Dion et al., 2009).
(2005) demonstrated an impaired constitutive extracellular secretion of mutant SOD1 in NSC-34 cells that induces frequent cytoplasmic inclusions and protein insolubility. These data link the deficient secretion of mutant SOD1 with intracellular protein aggregates and toxicity in NSC-34 cells. In addition, these authors showed that in a transgenic rat model of ALS the chronic intraspinal infusion of exogenous human wt-SOD1 significantly delayed disease progression suggesting a novel extracellular role for SOD1 in ALS; therefore extracellular delivery of human wt-SOD1 could improve clinical disease in transgenic ALS rats supporting a novel extracellular role for mutant and wt-SOD1 in ALS pathogenesis and therapy, respectively.
In addition, in transgenic mice, carrying SOD1 mutations, toxic effects to motor neurons by microglia activation were observed (Urushitani et al., 2006; Zhao et al., 2010). The microglia cells can carry out an important role in ALS progression (Pramatarova et al., 2001) since microglia activation can be observed before neuron loss in transgenic mice expressing human SOD1 mutants (Alexianu et al., 2001).


Thanks for sharing your info. I really appreciate your efforts and I am waiting for your further write ups thanks once again. Tandie Lazarus Hughes